Description
Details
Composition
Each vial contains
- Stabilizer: Human serum albumin 0.5mg (Korean Minimum Requirement for Biopharmaceutical Product)
- Isotonic agent: Sodium chloride (USP) 0.9mg
* One unit(U) of NABOTA corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice.
Description
It appears as a white to yellowish, vacuum-dried powder for injection in a colorless and transparent vial. It should become colorless transparent liquid when dissolved in the diluent (physiological saline solution).
Why Choose NABOTA?
Patented Technology
NABOTA was developed with 30 years of experience in biotechnology.
NABOTA ensures the quality of international standards via its own patented purification process(Patent registration KR 10-1339349 in 2013)
Reduced Impurity
NABOTA is a highly purified product manufactured with a patented purification process from which impurities are removed as much as possible.
Overseas Expansion
Excellence in the quality of NABOTA has led to contracts to export the finished product to about 90 countries including the United States and 28 European countries.
Proven Efficacy & Safety
NABOTA demonstrates a rapid onset of improvement effects in glabella lines.
Rapid improvement in glabella lines
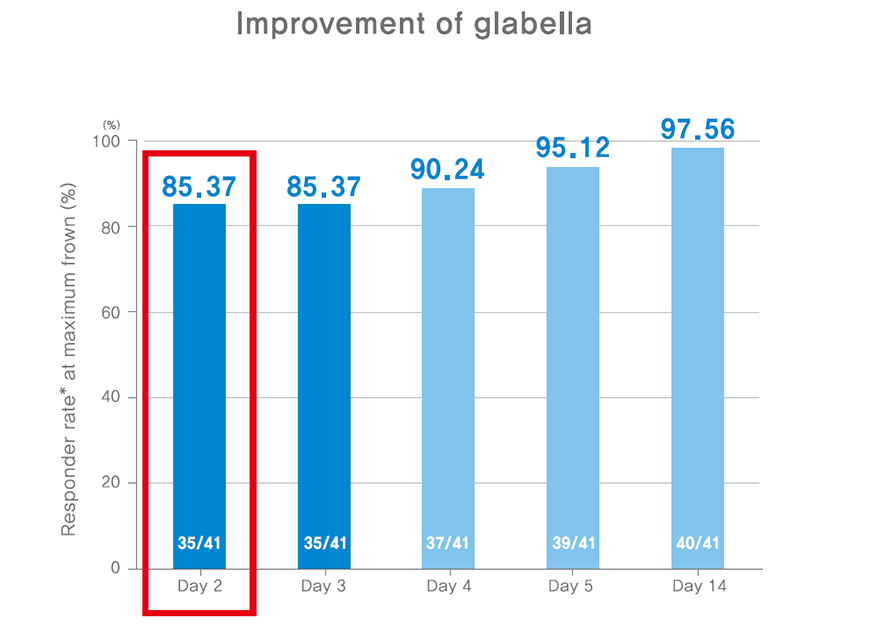
Responder rate: The number of the patient or the rate of the entire patients whose evaluation score decreased over one point.
Method of evaluation: At maximal frown, independent physician grades the level of glabella lines according to 0 point (none), 1 point (mild level), 2 point (moderate level), 3 point (severe level).
Methodology: single group, open, single center, phase IV
intramuscular inject of total 20U in 5 sites (0.1mL(4U) per site) in the glabella lines
evaluate improvement rate and safety in glabella lines at 2, 3, 4, 5, 14 days after injection.
Subjects: participants with glabella lines of at least moderate severity at maximum frown (n=44)
Results: NABOTA shows improvement effects in glabella lines after two days of injection. (Improvement rate: 85.37%)
NABOTA is effective in improving glabella lines.
Improvement in glabella lines
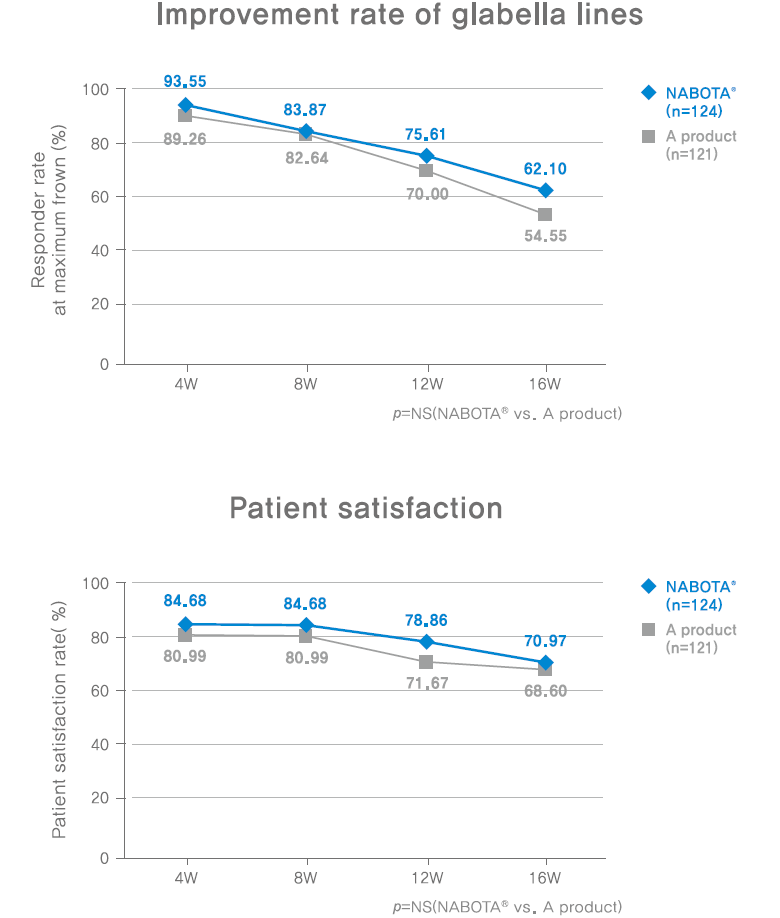
Responder rate: percentage of subjects with a score of none(0) or mild(1).
Method of evaluation: Investigator assessed the glabella line severity at maximum frown using a four-point scale: 0=none, 1=mild, 2=moderate, 3=severe.
Satisfaction rate: percentage of subjects who scored more than six-point(satisfied or very satisfied)
Method of evaluation: satisfaction was assessed using the following seven-category scale: 1=very dissatisfied, 2=dissatisfied, 3=somewhat dissatisfied, 4=indifferent, 5=somewhat satisfied, 6=satisfied, 7=very satisfied.
Methodology: prospective, double-blinded, randomized, active-controlled, phase III study.
instramuscular injection of total 20U in 5 sites(0.1mL(4U) per site) of glabella line and evaluate 4, 8, 12, 16 weeks after injection.
Subjects: participants with glabella lines of at least moderate severity at maximum frown (N=268)
Results:
- NABOTA demonstrates an improvement effect in glabella lines and high patient satisfaction.
- NABOTA demonstrates to be non-inferior when compared with A product.
NABOTA did not form antibody in phase III
Antibody is not formed in glabella lines.
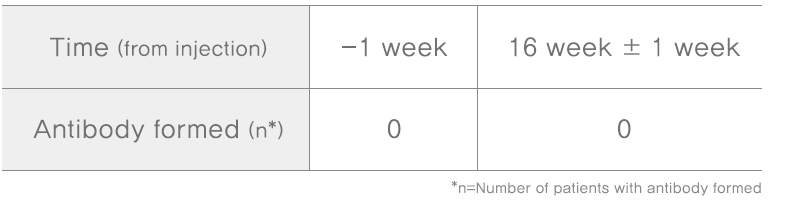
Methodology: active-controlled, double-blind, randomized, multicenter, phase III upon screening visit and closing visit of patients, to collect 12mL blood each, separate serum and analyze antibody formation with mouse bioassay(MBA)
Subjects: participants with glabella lines of at least moderate severity at maximum frown(n=135)
Results: Tested for 16 weeks to check if the patients have antibody after NABOTA injection, all patients did not have new antibody after 16 weeks with small doses of 20 units.
Antibody is not formed in post-stroke upper limb spasticity.
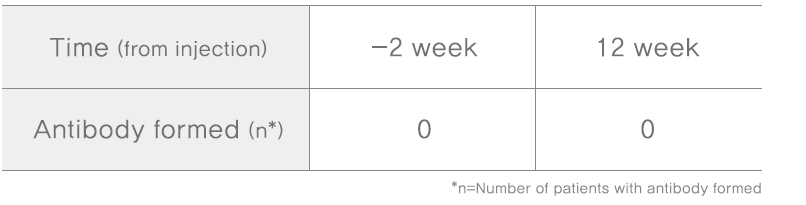
Methodology: active-controlled, double-blind, randomized, multicenter, phase III upon screening visit and closing visit of patients, to collect 12mL blood each, separate serum and analyze antibody formation with mouse bioassay(MBA)
Subjects: adult participants with post-stroke upper limb spasticity(n=197)
Results: Tested for 12 weeks to check if the patients have antibody after NABOTA injection, all patients did not have new antibody after 12 weeks with larger doses of 360 units,
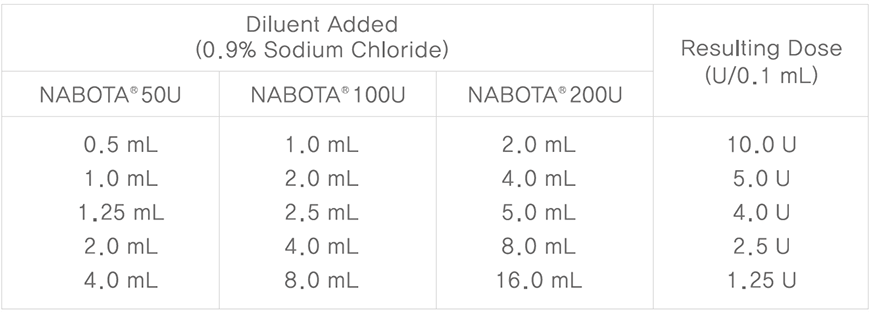
Reconstitution and Dilution Technique
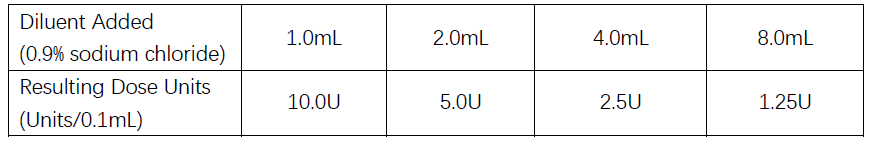
- Using an appropriate syringe, draw nonpreserved sterile saline (see dilution table below). 0.9% Sodium Chloride is the recommended diluent.
- Insert the needle and slowly inject the saline into the NABOTA vial in which vacuum is present.
- Inject slowly and avoid forming bubbles.
- Gently mix lyophilized NABOTA until it is completely clear and no particles are visible.
- Record the date and time of reconstitution. The solution should be administered within 24 hours after reconstitution.
- Reconstituted product should be stored in a refrigerator (2~8℃).
Stability after Dilution
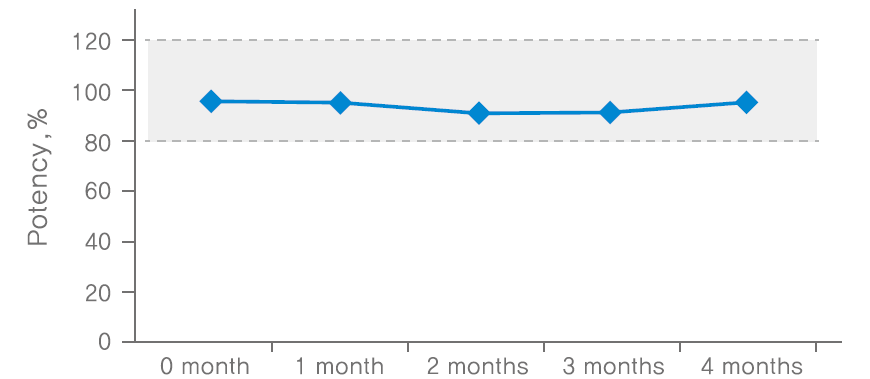
Stability at freezing temperature after dilution (-15~-25℃)
Stability at room temperature after dilution (15~25℃)
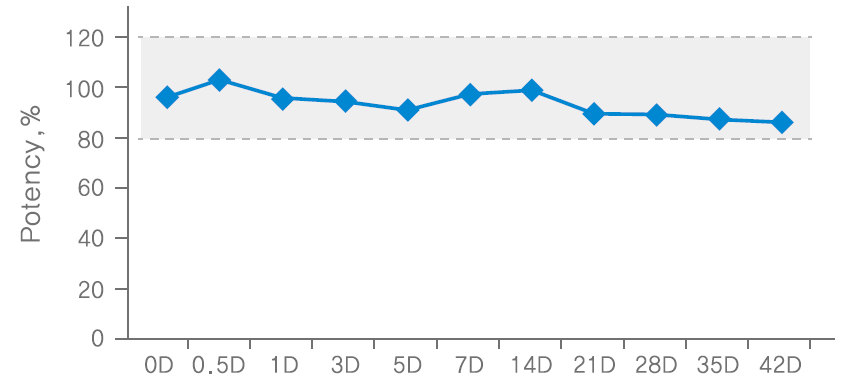
Stability after repetitive freezing/thawing after dilution (-15~-25℃/2~8℃)
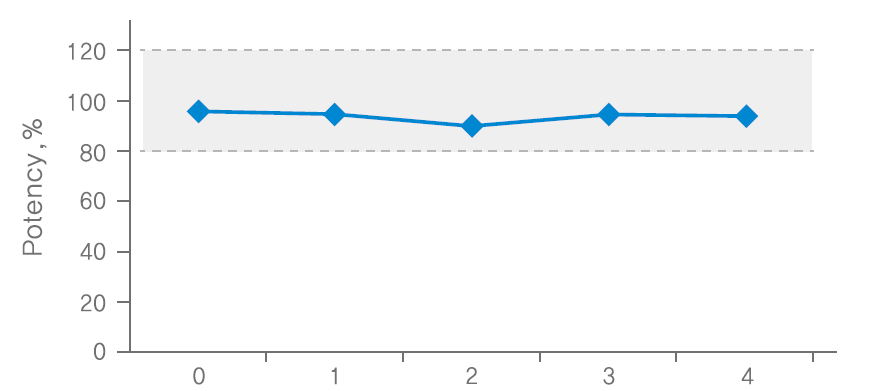
Cycle: regarded freezing (7days=-15~-25, 7days) and thawing(2~8℃, 48 hours) of solution as 1 cycle, repeated the cycle 4 times.
Methodology: conducted potency test using 3 batches of NABOTA 100U finished products, and measured the average value as potency. confirmed the stability of 100U NABOTA diluted in saline through animal potency test at room temperature, freezing and repetition of freezing/thawing conditions.
Results: The potency of NABOTA was proven to be effective when stored in a freezer for up to 4 months, room temperature for up to 5 weeks and repetition of freezing/thawing carried out up to 4 times after reconstitution.
Indication and Usage
Temporary improvement of forehead lines associated with corrugator and/or procerus muscle activity in adult patients between 20 to 65 years old
Upper limb spasticity after stroke in adult patients above 18 years old
Dilution Method
Storage
The unopened lyophilized vial should be stored in a freezer(blow -5℃) or refrigerator (2~8℃).
How supplied
NABOTA is supplied in a single-use vial.
Expiration
The shelf-life of NABOTA is 36 months from the manufacturing date.