Description
Details
Composition
Each vial contains
- Active ingredient: Clostridium botulinum toxin type A 200 units(U) (Korean Minimum Requirement for Biopharmaceutical Product)
- Stabilizer: Human serum albumin 1.0mg (Korean Minimum Requirement for Biopharmaceutical Product)
- Isotonic agent: Sodium chloride (EP) 1.8mg
* One unit(U) of Neuronox corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice.

Description
It appears as a lyophilized white powder for injection in a colorless transparent vial.
Proven Efficacy & Safety
The efficacy and the safety of Neuronox are proved to be comparable to Botox(Allergan Inc.)’s in various clinical studies.
Glabellar Frown Lines
The comparative clinical study for glabellar frown lines1) with Neuronox vs. Botox: Comparable Efficacy and Safety.
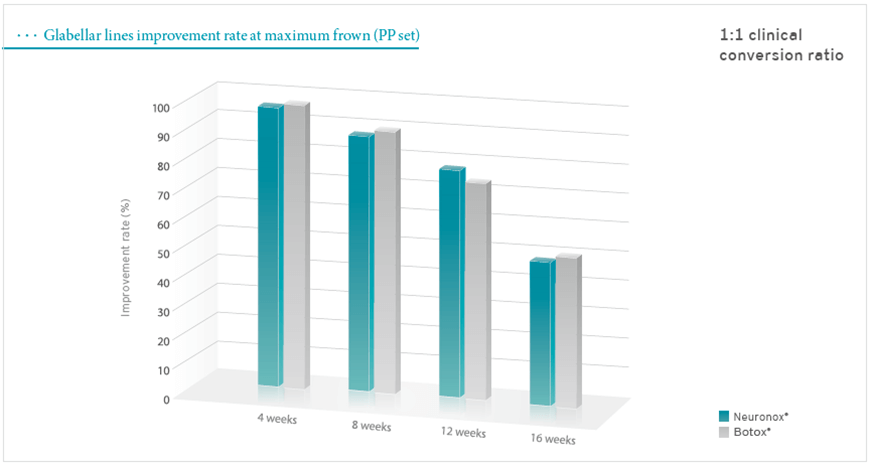
Methodology: Multicenter, double-blind, randomized, parallel design, active-controlled phase III clinical study. Each patient was randomly assigned to receive 20U (0.5ml) of either Neuronox(n=142) or Botox(n=146). The total dose was distributed over 5 injection points into corrugators and procerus. Results of the treatment were evaluated at 4-week intervals: up to 16 weeks.
Subjects: 314 healthy adult patients (aged between 20 to 65) with moderate or severe glabellar lines at maximum frown. *four-point scale (0=none, 1=mild, 2=moderate, 3=severe)
Results: Neuronox was proved its non-inferiority to Botox in this clinical study. Therefore, Neuronox is effective and safe for the treatment of glabellar frown lines.
SAFETY
In this study, 26.92% of patients treated with Neuronox and 22.29% of patients received Botox experienced adverse events. There was no statistical difference in the incidence and the severity of adverse events between two products (p=0.3416).
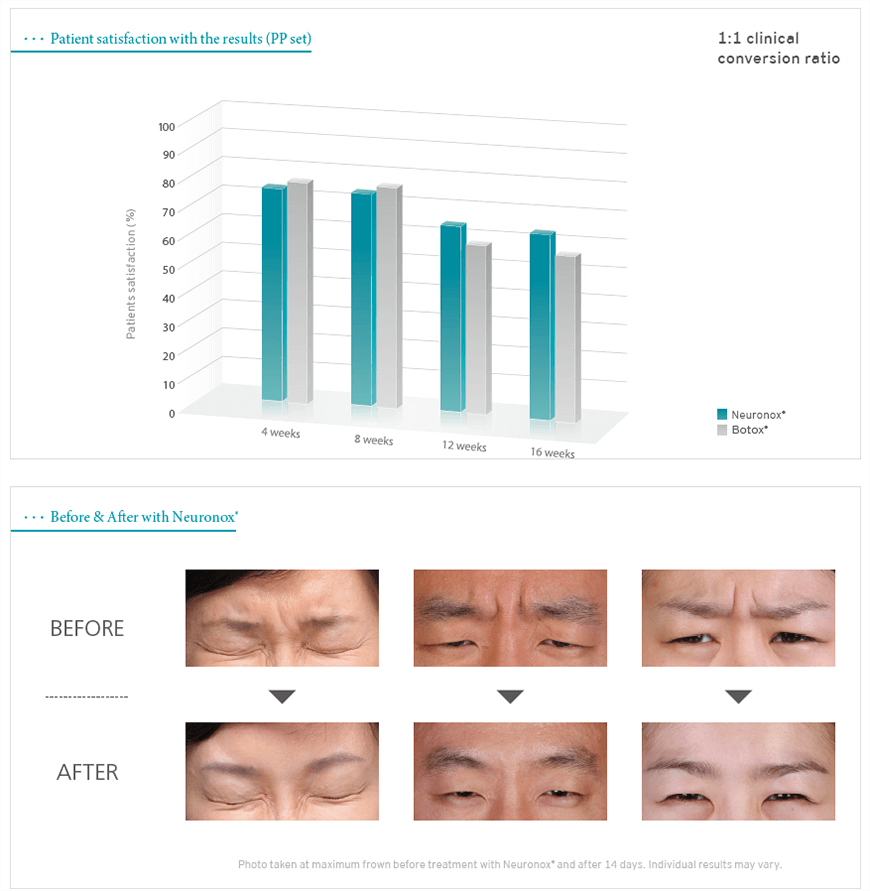
The comparative clinical study for glabellar frown lines with Neuronox vs. Botox: More satisfaction
Essential Blepharospasm
The comparative clinical study for essential blepharospasm with Neuronox vs. Botox: Comparable Efficacy and Safety
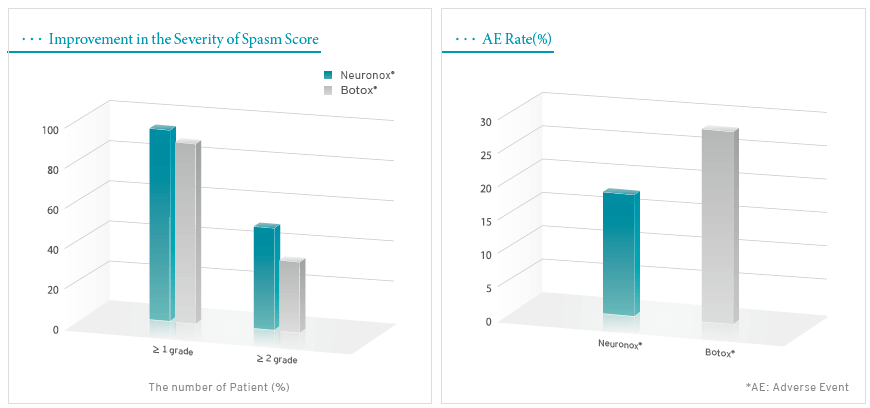
Methodology: Multi-center, double-blinded, randomized, active-controlled, parallel designed, phase Ⅲ clinical study.
Subjects: 60 patients diagnosed as essential blepharospasm (Neuronox n=31 / Botox n=29).
Results: The efficacy of Neuronox was not inferior to Botox in this clinical study. No difference was noted in the frequency of adverse event.
Neuronox can be safely used as an alternative to Botox treatment at 1:1 equivalence.
Equinus Deformity in Cerebral Palsy
The comparative clinical study for equinus deformity in cerebral palsy with Neuronox vs. Botox: Comparable Efficacy and Safety
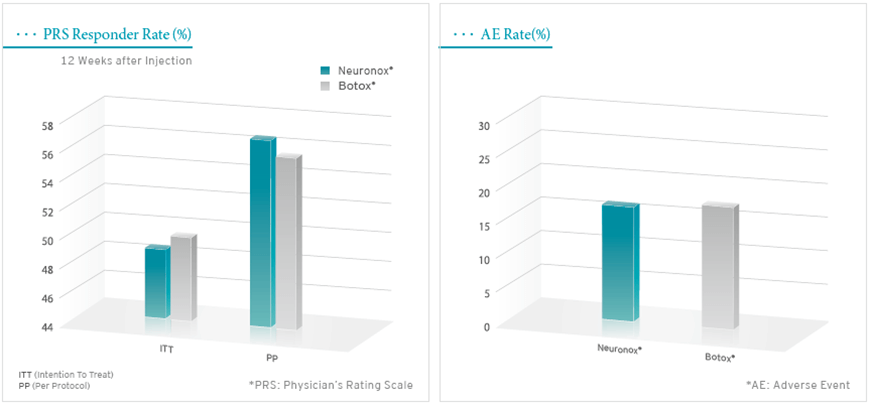
Methodology: Multi-center double-blinded, randomized, active-controlled, parallel designed, phase Ⅲ clinical study
Subjects: 119 pediatric patients diagnosed as spastic cerebral palsy with equinus foot deformity (Neuronox n=60 / Botox n=59)
Results: Neuronox was not inferior to Botox in this clinical study. No differences were noted in the frequency of adverse event.
Neuronox can be safely used as an alternative to Botox treatment.
Indication and Usage
1. Neuronox is indicated for the treatment of benign essential blepharospasm in patients 18 years of age and above.
2. Neuronox is indicated for the treatment of equinus foot deformity due to spasticity in pediatric cerebral palsy patients 2 years of age and above.
3. Neuronox is indicated for the treatment of temporarily improve moderate to severe glabellar wrinkles related to eyebrow wrinkle muscle (corrugator muscle) and/or procerus muscle activity in adults aged from 20 to 65.
4. Neuronox is indicated for the treatment of Muscle spasticity: upper limb local muscle spasticity related to stroke in adults aged 20 or more
Storage
The unopened lyophilized vial should be stored in a freezer(blow -5℃) or refrigerator (2~8℃).
How supplied
Neuronox is supplied in a single-use vial.
Expiration
The shelf-life of Neuronox is 36 months from the manufacturing date.
Specification
Specification
| Voltage | No |
|---|---|
| Materials | No |
| Package Size(cm) | No |
| Gross Weight | 0.1KG |
| ODM&OEM | No |
Shipping&Payment
Shipping
Delivery country: currently we only deliver to the USA, Canada, Australia, the UK, New Zealand. EU, Japan, South Korea, Singapore, China, Malaysia and Vietnam.
Importation duty: iBeautyMachine.com covers importation duty.
Excludes: special items such as gas or liquid.
Remote regions may cause extra for delivery to the door.
Please refresh the checkout page if you change the cart in case the free shipping option doesn't show up.
NOTE:
Warehouse working time: 9:00 am~ 6:pm (Monday to Friday; GMT+8).
Payment
Please note: We DO NOT accept Credit Card payments for product value of a single unit over 1,500 USD.
If you are not happy with the order and the product, you can ask for a refund after receiving the package. Our customer support will assist you with it.
We do not add taxes, VAT, or any other hidden charges. You pay us what you see on your invoice, for example, Goods Subtotal + Shipping Costs (does not include duties). Please find out as much as you can about import taxes in your own country before purchasing an item. In special cases, you may need to pay import duties on certain goods. You can contact us for further assistance on any of this.
Reviews
Tags
Product Questions
You may also be interested in the following product(s)