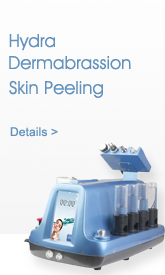
Description
Details
Introduction
Kairax is a hyaluronic acid-based medical device that involves maximized safety, convenience, and effectiveness based on an excellent technology. It is also fully CE approved and contains lidocaine to minimize comfort upon administration. Kairax can be injected dermally and can reach the subcutaneous layer of the skin to improve and correct wrinkles, hollow areas, scars, and facial lines. It also produces natural hydration to the skin and has excellent moisture retention. Because Kairax is formulated with hyaluronic acid, it is 100% biodegradable in the body and shows an excellent safety rate.
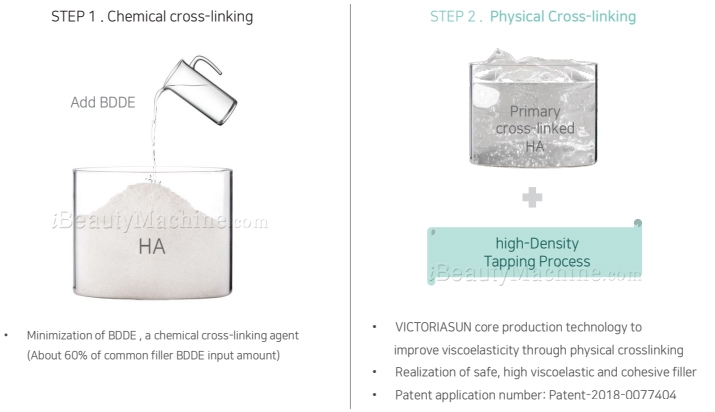
Kairax Dermal Filler is manufactured by VictoriaSun with their core h-DTP technology, where in it is a 2-step process involving chemical cross-linking, which is the first step, then a physical cross-linking as the second and last step of the procedure. This technology minimizes the presence of BDDE, a crosslinker agent, thus earning a certification for its safety. In addition to stabilization of the HA, a high-Density Tapping Process is used to improve its viscoelasticity and cohesion. These properties only shows that Kairax can efficiently bring volume to the hollow and depressed parts of the face while also maintaining the results for a long time. Kairax is perfect for the temporary correction of facial wrinkles to maintain one’s youth and beauty.
What is in the box?
- 1 x 1.0 mL pre-filled syringe
- 2 sterile needles, 30G
Kairax Product Line

Why Kairax?
1. Technology
Kairax is completely biodegradable indicating its high purity which results in minimized side effects. Through a strict purification process, Kairax are free of impurities, such as BDDE residues and endotoxins that can cause adverse effects. The protein residue from the HA material displays a below 0.1% value. Kairax only uses EDQM listed materials to ensure safety during use.
a. h-DTP
b. Degradation Test
Change over time after injecting 1 ml of hyaluronidase 750 IU into 0.5 ml of KAIRAX

2. Safety
Kairax is completely biodegradable indicating its high purity which results in minimized side effects. Through a strict purification process, Kairax are free of impurities, such as BDDE residues and endotoxins that can cause adverse effects. The protein residue from the HA material displays a below 0.1% value. Kairax only uses EDQM listed materials to ensure safety during use.
a. Purification Process
Impurities such as non-crosslinked BDDE (unreacted BDDE & pendant BDDE) residues and endotoxin residues were minimized through a strict purification process.
- BDDE residue: Below detection limit
- Endotoxin residue: Below 0.1Eu/ml
- Protein residue from HA raw material: Below 0.1%

b. Raw Materials
Pay extra attention to quality control, by using EDQM listed materials such as
HA (150kDA heavy-molecular raw material), lidocaine, and BDDE to ensure safety.

3. Convenience – Extrusion Force
In the clinical tests, Kairax Fillers show exceptional extrusion force with uniform distribution of the gel once injected. It ensures a stable and smooth administration of the filler providing convenience to the doctor and comfort for the patient. Kairax Dermal Fillers are also available in a wide range of applications which can broaden the options of the doctor and decide which suits best for the patient’s individual needs.
The KAIRAX extrusion force, indicated by a purple line, is a straight line since the initial rise. This means that when the filler is first injected, the extrusion force is high, but the injection pressure thereafter is uniform. Equal pressure increases the convenience of the procedure with a stable and smooth shooting.

4. Effectiveness – Viscoelasticity
Kairax Fillers maintains an excellent volume for a long time due to its high concentration of hyaluronic acid and their gel’s cohesivity.
KAIRAX Sub-Q has a viscoelasticity of 427.8 Pa, which belong to a filler with high viscoelasticity. The high viscoelasticity of KAIRAX maintains the excellent volume effect for a long time.

Application
Kairax Fine filler has a low viscoelasticity and is best suited for treating fine wrinkles in areas such as the lips, crows feet, and periorbital lines.
Injection Depth: Superficial dermis
Duration of effect: 3 – 6 months
Application Areas:
• Periorbital line
• Crow’s feet
• Lip contour

Contraindicated
Celosome Dermal Filler must not be used in:
- Women who are pregnant or breastfeeding
- People under age of 18
- Patients who are known to demonstrate hypersensitivity to hyaluronic acid.
- Patients who are known to demonstrate hypersensitivity to lidocaine
- Patients who tend to develop hypertrophic scarring.
- Immediately before or post laser therapy, chemical peeling or derma-brasion.
- Areas presenting cutaneous inflammation or signs of infection.
Specification
Specification
| Package List |
|
|---|
Shipping&Payment
Shipping
Delivery country: currently we only deliver to the USA, Canada, Australia, the UK, New Zealand. EU, Japan, South Korea, Singapore, China, Malaysia and Vietnam.
Importation duty: iBeautyMachine.com covers importation duty.
Excludes: special items such as gas or liquid.
Remote regions may cause extra for delivery to the door.
Please refresh the checkout page if you change the cart in case the free shipping option doesn't show up.
NOTE:
Warehouse working time: 9:00 am~ 6:pm (Monday to Friday; GMT+8).
Payment
Please note: We DO NOT accept Credit Card payments for product value of a single unit over 1,500 USD.
If you are not happy with the order and the product, you can ask for a refund after receiving the package. Our customer support will assist you with it.
We do not add taxes, VAT, or any other hidden charges. You pay us what you see on your invoice, for example, Goods Subtotal + Shipping Costs (does not include duties). Please find out as much as you can about import taxes in your own country before purchasing an item. In special cases, you may need to pay import duties on certain goods. You can contact us for further assistance on any of this.
Reviews
Tags
Product Questions
You may also be interested in the following product(s)